Abstract
BACKGROUNG. Fixed duration venetoclax-based therapy, either in combination with BTK inhibitors or anti-CD20 monoclonal antibody, is a highly active front-line treatment in patients with chronic lymphocytic leukemia (CLL). However, patients with TP53 abnormalities may early relapse after the end of treatment. Continuous treatment with BTK inhibitor is a valid option in TP53 disrupted patients, counteracted by a relatively high rate of discontinuation due to adverse events. In the phase II study investigating venetoclax in TP53-disrupted CLL patients, only 5 patients were treatment naive (TN) and 4/5 were remission-free after a median follow-up of 70months (Stilgenbauer S, EHA2022).
AIM. The aim of this retrospective study is to describe the efficacy and discontinuation rate of continuous venetoclax in TN CLL patients with TP53 disruption in the real-life setting.
METHODS. Medical charts of CLL patients from 16 centers belonging to the Italian Campus CLL network were retrospectively reviewed to identify CLL with del17p13(cut-off 10%) and/or TP53 mutation (TP53m) treated front-line with continuous venetoclax. The primary endpoint was the rate of treatment discontinuation. Secondary endpoints were overall response rate (ORR), measurable residual disease (MRD) by flow-cytometry, progression-free survival (PFS), overall survival (OS) and comparison with a cohort of 100 TN patients treated with ibrutinib (updated from Visentin A, AJH2022). CLL diagnosis and response assessment were carried out according to the iwCLL2018 guidelines. Adverse events were classified according to the CTCAEv5.0 grading. Statistical analyses were performed with Prism7.
RESULTS. Thirty-four TN CLL with TP53 abnormalities were recruited. The median age was 69 years (range 46-84), median CIRS score was 3.5 (range 0-12), 24% had a creatinine clearance <60ml/min. LDH and β2-microglobulin were increased in 38% and 48% of patients. Seventy-one % of patients were IGHV unmutated, 79% harbored del17p13 and 74% TP53m. According to the tumor lysis syndrome (TLS) risk score, 12% were classified at low-risk, 35% intermediate-risk and 53% at high-risk of TLS. Ninety-nine patients were hospitalized during the ramp-up phase while the remaining in the out-patient clinics. No patient developed clinical or biochemical TLS. Eighty-two % of patients were able to reach 400mg of venetoclax.
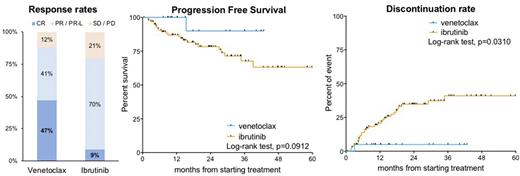
The best ORR was 85%, including 50% complete responses (CR) and 35% partial responses (PR) (Fig. 1). The median time to CR was 14.9 months. MRD was assessed in the peripheral blood of 14 patients, achieving undetectable MRD4 in 6 (43%), detectable MRD4 in 6 (43%) and detectable MRD2 in 2 (14%) patients. After a median follow-up of 20 months, 9 patients decreased the venetoclax dose and 3 discontinued therapy (thrombocytopenia 1 and Richter transformation 2). The median PFS and OS were not reached. The 12 and 24-month PFS were 96% and 83%, respectively (Fig. 1). All patients were alive after 12 months and the estimated 24-month OS was 88%. Regarding safety, we recorded of grade ≥3 (G≥3) adverse events in 23 (67%) patients, including hematological toxicity in 14 (11 neutropenia, 2 anemia, 1 thrombocytopenia), second primary malignancies in 3, diarrhea in 3, infections in 2 and major bleeding in 2. Atrial fibrillation occurred in 2 patients.
Compared to patients treated with ibrutinib (n=100 , comparable for age and comorbidities, median follow-up 24months), we observed a higher CR rate (50% vs 9%, p<0.0001), a trend for a longer PFS (p=0.10) and a lower incidence of discontinuation (24-month 14% vs 35%, p=0.0194) in patients receiving venetoclax (Fig. 1). We also observed a higher rate of all G≥3 events (67% vs 22%, p<0.0001) and severe neutropenia in patients treated with venetoclax (32% vs 4%, p<0.0001), but a lower rate of infections (6% vs 22%, p=0.0386) and cardiovascular events (6% vs 14%, p=0.3573).
CONCLUSION. To our knowledge, we report the largest real-life study of continuous venetoclax in TN CLL patients with TP53 abnormalities, indicating a high efficacy of venetoclax in this subset of patients, not previously studied systematically in clinical trials. Only one patient discontinued venetoclax due to toxicity. The use of continuous venetoclax in TN CLL patients with TP53 disruption deserve further investigation, as it can be an effective alternative option beside BTK inhibitor.
Disclosures
Visentin:Janssen, Abbvie, CSL Behring, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Scarfò:BeiGene, Janssen: Other: Travel Grant; AbbVie, AstraZeneca, Janssen, Beigene: Honoraria; Octopharma: Speakers Bureau. Reda:Janssen, Abbive, Astra Zeneca, Beigene: Consultancy. Ferrarini:Abbvie: Research Funding. Sanna:Abbvie: Honoraria, Other: travel, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: travel, Speakers Bureau; Astra Zeneca: Honoraria, Speakers Bureau. Murru:Janssen: Research Funding; Janssen, Abbvie, Astra Zeneca: Honoraria, Other: travel. Rigolin:Abbvie: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; Gilead: Honoraria, Research Funding. Molica:AbbVie, Janssen, Astra-Zeneca: Consultancy, Honoraria. Ghia:Janssen: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Lilly/Loxo: Consultancy, Honoraria; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal